DISCOVERIES REPORTS (ISSN 2393249X), 2022, volume 5
REVIEW ARTICLE
CITATION: Stilpeanu RI, Stercu AM. Monkeypox, a current global health issue: brief review on prevention and treatment. Discoveries Reports 2022; 5(2): e33. DOI: 10.15190/drep.2022.7
Monkeypox, a current global health issue: brief review on prevention and treatment
Ruxandra Ilinca Stilpeanu 1,2, *, Ana Maria Stercu 1,2, *
1 Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
2 Victor Babes National Institute of Pathology, Bucharest, Romania
* Corresponding authors:
Ruxandra Ilinca Stilpeanu, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania, ruxandra.stilpeanu@gmail.com;
Ana Maria Stercu, Carol Davila University of Medicine and Pharmacy, Bucharest,
Romania, stercu.anamaria@yahoo.com
Abstract
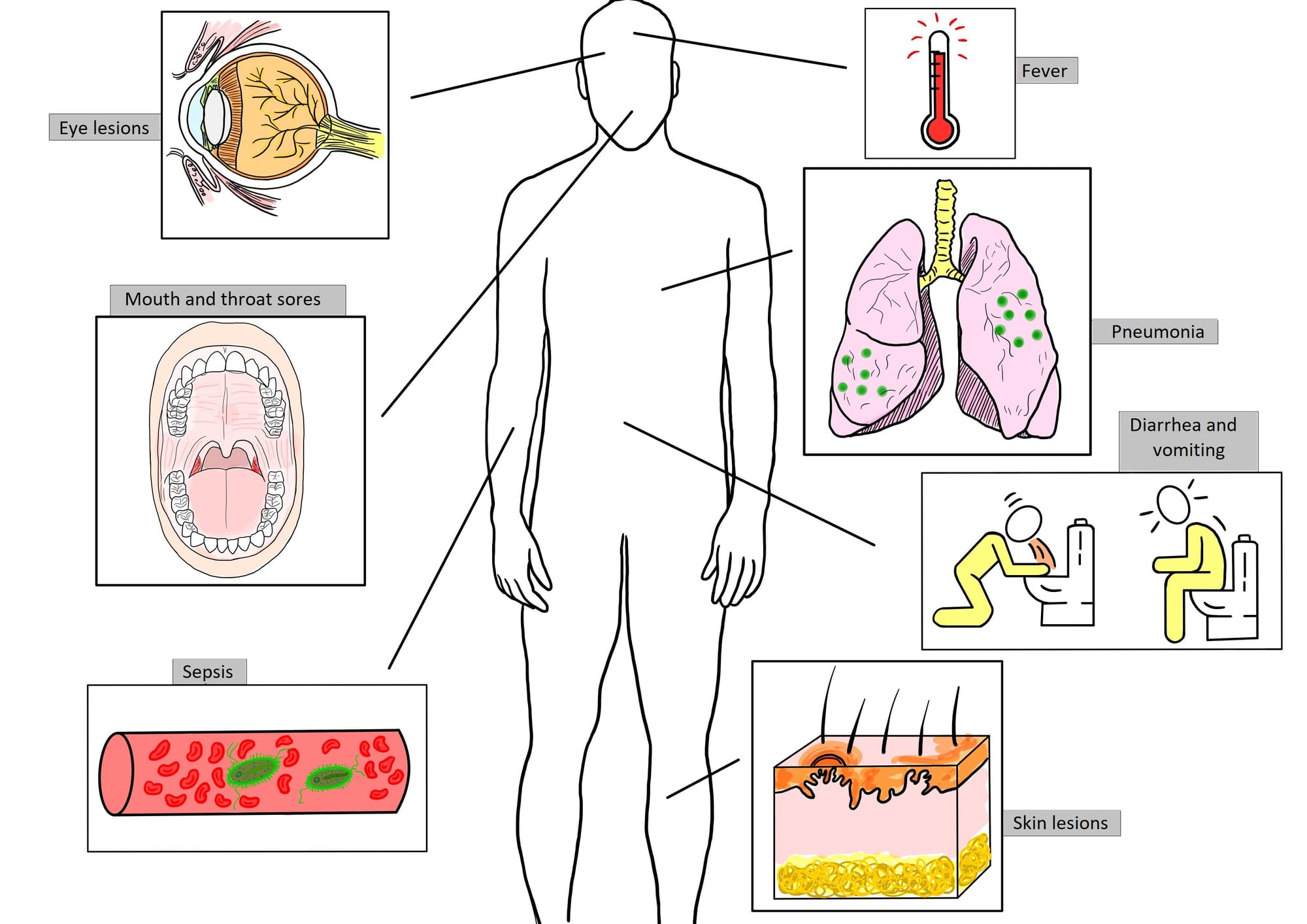
While the COVID-19 pandemic seems to fade away, humanity is currently facing another health challenge risen by the zoonotic orthopoxvirus called monkeypox, with a number of cases that seems to be decreasing for the first time since the beginning of 2022, when the pandemic commenced. This review begins by describing the actual global picture of this phenomenon and progresses, depicting the potential measures of prevention and treatment among monkeypox patients. After various reports that proved the high transmission of this virus through direct contact with infected patients, the actions that are being taken worldwide revolve around prevention among individuals at high risk, with measures of prophylaxis before and after exposure, while covering immunization with vaccines once used for smallpox (ACAM2000, JYNNEOS or Imvamune). Furthermore, healthcare specialists should address treating infected patients with antiviral therapy, such as tecovirimat, brincidofovir and cidofovir, and without overlooking supportive care and the option of Vaccinia Immune Globulin, the latter still being debatable due to its possible contraindications.
Access FULL text of the manuscript here: FULL ARTICLE (PDF)
REFERENCES
1. Multi-country outbreak of monkeypox, External situation report #5 - 7 September 2022. Accessed September 22, 2022. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--5---7-september-2022
2. Olson VA, Laue T, Laker MT, et al. Real-Time PCR System for Detection of Orthopoxviruses and Simultaneous Identification of Smallpox Virus. J Clin Microbiol. 2004;42(5):1940-1946. doi:10.1128/JCM.42.5.1940
3. Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86(Pt 10):2661-2672. doi:10.1099/VIR.0.81215-0
4. Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic Variability of Monkeypox Virus among Humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20(2):232. doi:10.3201/EID2002. 130118
5. Wilson ME, Hughes JM, McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58(2):260-267. doi:10.1093/CID/CIT703
6. Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378. doi:10.1136/BMJ-2022-072410
7. Grant R, Nguyen LBL, Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98(9):638. doi:10.2471/BLT.19.242347
8. Treatment Information for Healthcare Professionals | Monkeypox | Poxvirus | CDC. Accessed September 22, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
9. Vaccines | Smallpox | CDC. Accessed Sept. 22, 2022. https://www.cdc.gov/smallpox/clinicians/vaccines.html
10. Joint ECDC-WHO Regional Office for Europe Monkeypox Surveillance Bulletin: 21 September 2022. Accessed September 22, 2022. https://www.who.int/europe/publications/m/item/joint-ecdc-who-regional-office-for-europe-monkeypox-surveillance-bulletin--21-september-2022
11. Surveillance, case investigation and contact tracing for Monkeypox: Interim guidance. Accessed September 22, 2022. https://www.who.int/publications/i/item/WHO-MPX-Surveillance-2022.3
12. Moore MJ, Rathish B, Zahra F. Monkeypox. StatPearls. Published online July 16, 2022. Accessed September 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK574519/
13. Vaccination Strategies | Monkeypox | Poxvirus | CDC. Accessed September 22, 2022. https://www.cdc.gov/poxvirus/monkeypox/interim-considerations/overview.html
14. Fleischauer AT, Kile JC, Davidson M, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40(5):689-694. doi:10.1086/427805
15. Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and Treatment of Monkeypox. Drugs. 2022;82(9):957. doi:10.1007/S40265-022-01742-Y
16. Petersen BW, Harms TJ, Reynolds MG, Harrison LH. Use of Vaccinia Virus Smallpox Vaccine in Laboratory and Health Care Personnel at Risk for Occupational Exposure to Orthopoxviruses - Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2015. MMWR Morb Mortal Wkly Rep. 2016;65(10):257-262. doi:10.15585/MMWR.MM6510A2
17. Volz A, Sutter G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv Virus Res. 2017;97:187-243. doi:10.1016/BS.AIVIR.2016.07.001
18. Kennedy JS, Gurwith M, Dekker CL, et al. Safety and Immunogenicity of LC16m8, an Attenuated Smallpox Vaccine in Vaccinia-Naive Adults. J Infect Dis. 2011;204(9):1395. doi:10.1093/INFDIS/JIR527
19. Islam MdR, Hossain MdJ, Roy A, et al. Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: A rapid review on comparative benefits and risks. Health Sci Rep. 2022;5(5). doi:10.1002/HSR2.798
20. Centers for Disease Control and Prevention Center for Preparedness and Response Monkeypox Outbreak: Updates on the Epidemiology, Testing, Treatment, and Vaccination Clinician Outreach and Communication Activity (COCA) Call. Published online 2022.
21. Monkeypox and Smallpox Vaccine Guidance | Monkeypox | Poxvirus | CDC. Accessed September 22, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html
22. Badell ML, Meaney-Delman D, Tuuli MG, et al. Risks associated with smallpox vaccination in pregnancy: A systematic review and meta-analysis. Obstetrics and Gynecology. 2015;125(6):1439-1451. doi:10.1097/AOG.0000000000000857
23. Monkeypox | Poxvirus | CDC. Accessed September 23, 2022. https://www.cdc.gov/poxvirus/monkeypox/index.html
24. Farahat RA, Abdelaal A, Shah J, et al. Monkeypox outbreaks during COVID-19 pandemic: are we looking at an independent phenomenon or an overlapping pandemic? Ann Clin Microbiol Antimicrob. 2022;21(1):1-3. doi:10.1186/S12941-022-00518-2/FIGURES/1
25. Bunge EM, Hoet B, Chen L, et al. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2). doi:10.1371/JOURNAL.PNTD.0010141
26. Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773-780. doi:10.1086/505880
27. Gross E. Update on emerging infections: news from the Centers for Disease Control and prevention. Update: Multistate outbreak of monkeypox--Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. Ann Emerg Med. 2003;42(5):660-662; discussion 662-4. doi:10.1016/S0196064403008199
28. Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350(4):342-350. doi:10.1056/NEJMOA032299
29. Reynolds MG, McCollum AM, Nguete B, Lushima RS, Petersen BW. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017;9(12). doi:10.3390/V9120380
30. Wilson ME, Hughes JM, McCollum AM, Damon IK. Human Monkeypox. Clinical Infectious Diseases. 2014;58(2):260-267. doi:10.1093/CID/CIT703
31. Nalca A, Livingston VA, Garza NL, et al. Experimental infection of cynomolgus macaques (Macaca fascicularis) with aerosolized monkeypox virus. PLoS One. 2010;5(9):1-12. doi:10.1371/JOURNAL.PONE.0012880
32. Nipp RD, Kelley MJ, Williams CD, Kamal AH. Evolution of the Quality Oncology Practice Initiative supportive care quality measures portfolio and conformance at a Veterans Affairs medical center. J Oncol Pract. 2013;9(3). doi:10.1200/JOP.2013.000923
33. Barnewall RE, Fisher DA, Robertson AB, Vales PA, Knostman KA, Bigger JE. Inhalational monkeypox virus infection in cynomolgus macaques. Front Cell Infect Microbiol. 2012;2:117. doi:10.3389/fcimb.2012.00117
34. Siegrist EA, Sassine J. Antivirals With Activity Against Monkeypox: A Clinically Oriented Review. Clinical Infectious Diseases. Published online July 29, 2022. doi:10.1093/CID/CIAC622
35. Russo AT, Grosenbach DW, Chinsangaram J, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. https://doi.org/101080/1478721020201819791. 2020;19(3):331-344. doi:10.1080/14787210.2020.1819791
36. Russo AT, Grosenbach DW, Brasel TL, et al. Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques. J Infect Dis. 2018;218(9):1490. doi:10.1093/INFDIS/JIY326
37. Hoy SM. Tecovirimat: First Global Approval. Drugs. 2018;78(13):1377-1382. doi:10.1007/S40265-018-0967-6
38. Russo AT, Grosenbach DW, Chinsangaram J, et al. An overview of tecovirimat for smallpox treatment and expanded anti-orthopoxvirus applications. Expert Rev Anti Infect Ther. 2021;19(3):331-344. doi:10.1080/14787210.2020.1819791
39. Mucker EM, Goff AJ, Shamblin JD, et al. Efficacy of Tecovirimat (ST-246) in Nonhuman Primates Infected with Variola Virus (Smallpox). Antimicrob Agents Chemother. 2013;57(12):6246. doi:10.1128/AAC.00977-13
40. Hutson CL, Kondas A v., Mauldin MR, et al. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere. 2021;6(1). doi:10.1128/MSPHERE.00927-20
41. Andrei G, Snoeck R. Cidofovir Activity against Poxvirus Infections. Viruses. 2010;2(12):2803. doi:10.3390/V2122803
42. Hiwarkar P, Amrolia P, Sivaprakasam P, et al. Brincidofovir is highly efficacious in controlling adenoviremia in pediatric recipients of hematopoietic cell transplant. Blood. 2017;129(14):2033-2037. doi:10.1182/BLOOD-2016-11-749721
43. Tippin TK, Morrison ME, Brundage TM, Momméja-Marin H. Brincidofovir Is Not a Substrate for the Human Organic Anion Transporter 1: A Mechanistic Explanation for the Lack of Nephrotoxicity Observed in Clinical Studies. Ther Drug Monit. 2016;38(6):777. doi:10.1097/FTD.0000000000000353
44. Detweiler CJ, Mueller SB, Sung AD, Saullo JL, Prasad VK, Cardona DM. Brincidofovir (CMX001) Toxicity Associated with Epithelial Apoptosis and Crypt Drop Out in a Hematopoietic Cell Transplant Patient: Challenges in Distinguishing Drug Toxicity from GVHD. J Pediatr Hematol Oncol. 2018;40(6):e364. doi:10.1097/MPH.0000000000001227
45. Berger M, Stiehm ER. From Subcutaneous to Intravenous Immunoglobulin and Back. Primary Immunodeficiency Disorders: A Historic and Scientific Perspective. Published online August 26, 2014:41-49. doi:10.1016/B978-0-12-407179-7.00023-0
46. Vaccinia Immune Globulin Intravenous (Human) | FDA. Accessed September 22, 2022. https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/vaccinia-immune-globulin-intravenous-human
47. Wittek R. Vaccinia immune globulin: current policies, preparedness, and product safety and efficacy. Int J Infect Dis. 2006;10(3):193-201. doi:10.1016/J.IJID.2005.12.001
48. Kitt E, Bassiri H. SMALLPOX (VARIOLA VIRUS). The 5-Minute Pediatric Consult, 8th Edition. Published online January 1, 2009:2089-2101. doi:10.1016/B978-1-4160-4044-6.50180-1
49. Nalca A, Rimoin AW, Bavari S, Whitehouse CA. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin Infect Dis. 2005;41(12):1765-1771. doi:10.1086/498155
50. Lai CC, Hsu CK, Yen MY, Lee PI, Ko WC, Hsueh PR. Monkeypox: An emerging global threat during the COVID-19 pandemic. J Microbiol Immunol Infect. Published online 2022. doi:10.1016/J.JMII.2022.07.004
51. Rabbi MF, Hami I, Islam MK, Akter T, Hossain MS, Rahaman MM. COVID-19 Pandemic in South Asia: Challenges and Mitigation. Discoveries Reports, 2021; 4: e18. DOI: 10.15190/drep.2021.3. Discoveries Reports. 2021;4:18. doi:10.15190/drep.2021.3
52. Fortner A, Schumacher D. First COVID-19 Vaccines Receiving the US FDA and EMA Emergency Use Authorization. Discoveries. 2021;9(1):e122. doi:10.15190/d.2021.1
53. Haq AU, Bashir I, Ikhlaq A, Arshad AR, Ijaz F, Aftab RK. A Systematic Review of COVID-19 Reinfections. Discoveries Reports. 2021;4:21. doi:10.15190/drep.2021.6
54. Khan M, Adil SF, Alkhathlan HZ, et al. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules. 2020;26(1). doi:10.3390/MOLECULES26010039
55. Arshad AR, Ijaz F, Siddiqui MS, Khalid S, Fatima A, Aftab RK. COVID-19 pandemic and antimicrobial resistance in developing countries. Discoveries. 2021;9(2):e127. doi:10.15190/D.2021.6
56. Lytras T, Tsiodras S. Lockdowns and the COVID-19 pandemic: What is the endgame? Scand J Public Health. 2021;49(1):37-40. doi:10.1177/1403494820961293
57. Walia N, Lat JO, Tariq R, et al. Post-acute sequelae of COVID-19 and the mental health implications. Discoveries. 2021;9(4):e140. doi:10.15190/D.2021.19
58. Rodriguez‐leyva D, Pierce GN. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients. 2021;13(6). doi:10.3390/NU13061752
59. Saha O, Rakhi NN, Sultana A, Rahman MdM, Rahaman MdM. SARS-CoV-2 and COVID-19: A Threat to Global Health. Discoveries Reports. 2020;3:e13. doi:10.15190/DREP.2020.7