DISCOVERIES REPORTS (ISSN 2393249X), 2023, volume 6
ORIGINAL ARTICLE
CITATION: Immune checkpoint inhibitors and predictive biomarkers in checkpoint inhibition therapy. Discoveries Reports 2023; 6: e39. DOI: 10.15190/drep.2023.3
Immune checkpoint inhibitors and predictive biomarkers in checkpoint inhibition therapy
Sebastian-Timotei Nicolae 1,2 *
1 Southern Denmark University, Institute of Molecular Medicine, Odense, Denmark
2 Carol Davila University of Medicine and Pharmacy, Bucharest, Romania
* Corresponding authors:
Sebastian-Timotei Nicolae, Carol Davila University of Medicine and Pharmacy, Bucharest Romania: Email: timoteisebastian.nicolae@gmail.co
Abstract
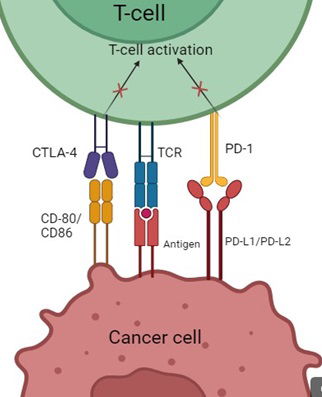
Cancer is one of the leading causes of death at a global scale. Many malignancies prove very hard to manage and current treatment methods, although effective to some extent, need improvement. Recently, Immune Checkpoints such as Programmed death receptor 1 (PD-1) and cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) have been a subject of great interest in cancer treatment. Blocking these receptors and their ligands enables the T-cells to recognize and destroy cancer cells easier, although this may not always be the case. Many studies have shown benefits of using immune checkpoint inhibitors (ICIs) over conventional chemotherapy, although there are some treatment-related adverse effects that are also to be taken into calculation. Most adverse reactions are autoimmune, given the fact that ICIs make the T-cells more reactive and block their co-inhibitory signals when activated. As with every treatment, predictive biomarkers for survival rate and response rate are very important, as such there has been a lot of research in order to find reliable markers that would allow an accurate estimate for the RR and OS of the patient. The most commonly used as of now are Tumor Mutation Burden, a marker that represents how many mutations cancer cells have suffered, as more of them would make the chances of immunogenic neoantigens being produced higher, Mismatch Repair Deficiency/ Microsatellite Instability, which is a marker that shows a tumor phenotype characterized by the production of immunogenic neoantigen, PD-L1 expression, which is a biomarker that has been linked with better response to ICI therapy, and Neutrophil to Lymphocyte Ratio, a value that expresses the balance between cancer induced inflammation and anti-tumor response of the body. In this review, I present FDA-approved checkpoint inhibitors and their applications, benefits and limitations of immune checkpoint blockade and predictive biomarkers and their accuracy and reliability. This manuscript offers insight into the uses and safety of checkpoint inhibitors as a cancer therapy, as well as the advantages and drawbacks of their most used biomarkers, thus allowing for a conclusive view on the topic. As such, Immune Checkpoint Inhibitors show great potential for cancer treatment. Predictive biomarkers for this new medicine are also promising and have proven reliable across many malignancies, although their ability to predict the response to ICI therapy can come under question in a number of scenarios.
Access FULL text of the manuscript here: FULL ARTICLE (PDF)
REFERENCES
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer Journal for Clinicians. 2022 72(1), 7–33. doi: 10.3322/CAAC.21708
2. Hui E. Immune checkpoint inhibitors. J Cell Biol. 2019 Mar 4;218(3):740-741 Epub 2019 Feb 13. PMID: 30760493; PMCID: PMC6400575, doi: 10.1083/jcb.201810035.
3. Wakeley ME, Gray CC, Monaghan SF, Heffernan DS, Ayala A. Check Point Inhibitors and Their Role in Immunosuppression in Sepsis. Crit Care Clin. 2020 Jan;36(1):69-88. Epub 2019 Oct 21. PMID: 31733683; PMCID: PMC6863093. doi: 10.1016/j.ccc.2019.08.006
4. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol. 2016 Dec;17(12):e542-e551. PMID: 27924752; PMCID: PMC5702534. doi: 10.1016/S1470-2045(16)30406-5.
5. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018 Dec 13;50(12):1-11 PMID: 30546008; PMCID: PMC6292890. doi: 10.1038/s12276-018-0191-1.
6. Gao X, McDermott DF. Ipilimumab in combination with nivolumab for the treatment of renal cell carcinoma. Expert Opin Biol Ther. 2018 Sep;18(9):947-957. Epub 2018 Aug 30. PMID: 30124333; PMCID: PMC6289271. doi: 10.1080/14712598.2018.1513485.
7. Ai L, Chen J, Yan H, He Q, Luo P, Xu Z, Yang X. Research Status and Outlook of PD-1/PD-L1 Inhibitors for Cancer Therapy. Drug Des Devel Ther. 2020 Sep 8;14:3625-3649. PMID: 32982171; PMCID: PMC7490077. doi: 10.2147/DDDT.S267433.
8. Kwok G, Yau TC, Chiu JW, Tse E, Kwong YL. Pembrolizumab (Keytruda). Hum Vaccin Immunother. 2016 Nov;12(11):2777-2789. Epub 2016 Jul 11. PMID: 27398650; PMCID: PMC5137544. doi: 10.1080/21645515.2016.1199310.
9. Rischin D, Migden MR, Lim AM, Schmults CD, Khushalani NI, Hughes BGM. Phase 2 study of cemiplimab in patients with metastatic cutaneous squamous cell carcinoma: primary analysis of fixed-dosing, long-term outcome of weight-based dosing. J Immunother Cancer. 2020 Jun;8(1):e000775. PMID: 32554615; PMCID: PMC7304829. doi: 10.1136/jitc-2020-000775
10. Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novák Z, Black D et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N Engl J Med. 2023 Jun 8;388(23):2145-2158. Epub 2023 Mar 27. PMID: 36972026. doi: 10.1056/NEJMoa2216334.
11. Kasherman L, Ahrari S, Lheureux S. Dostarlimab in the treatment of recurrent or primary advanced endometrial cancer. Future Oncol. 2021 Mar;17(8):877-892. Epub 2020 Nov 30. PMID: 33251877. doi: 10.2217/fon-2020-0655.
12. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Durvalumab. 2022 Jun 23. PMID: 31643480.
13. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012–. Avelumab. 2022 Jun 23. PMID: 31643848.
14. Inman BA, Longo TA, Ramalingam S, Harrison MR. Atezolizumab: A PD-L1-Blocking Antibody for Bladder Cancer. Clin Cancer Res. 2017 Apr 15;23(8):1886-1890. Epub 2016 Nov 30. PMID: 27903674. doi: 10.1158/1078-0432.CCR-16-1417
15. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125377s115lbl.pdf
16. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125554s112lbl.pdf
17. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf
18. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761097s014lbl.pdf
19. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761174s002lbl.pdf
20. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761069s033lbl.pdf
21. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761049s009lbl.pdf
22. U.S. Food and Drug Administration, accessed on 29.12.2023, also available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761034s043lbl.pdf
23. Franzin R, Netti GS, Spadaccino F, Porta C, Gesualdo L, Stallone G. The Use of Immune Checkpoint Inhibitors in Oncology and the Occurrence of AKI: Where Do We Stand? Front Immunol. 2020 Oct 8;11:574271. PMID: 33162990; PMCID: PMC7580288. doi: 10.3389/fimmu.2020.574271.
24. Nanda R, Chow LQ, Dees EC, Berger R, Gupta S, Geva R. Pembrolizumab in Patients With Advanced Triple-Negative Breast Cancer: Phase Ib KEYNOTE-012 Study. J Clin Oncol. 2016 Jul 20;34(21):2460-7. Epub 2016 May 2. PMID: 27138582; PMCID: PMC6816000. doi: 10.1200/JCO.2015.64.8931.
25. Heeke AL, Tan AR. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 2021 Jun;40(2):537-547. Epub 2021 Jun 8. PMID: 34101053; PMCID: PMC8184866. doi: 10.1007/s10555-021-09972-4.
26. Haugh AM, Probasco JC, Johnson DB. Neurologic complications of immune checkpoint inhibitors. Expert Opin Drug Saf. 2020 Apr;19(4):479-488. Epub 2020 Mar 11. PMID: 32126176; PMCID: PMC7192781. doi: 10.1080/14740338.2020.1738382.
27. .Jardim DL, Goodman A, de Melo Gagliato D, Kurzrock R. The Challenges of Tumor Mutational Burden as an Immunotherapy Biomarker. Cancer Cell. 2021 Feb 8;39(2):154-173. Epub 2020 Oct 29. PMID: 33125859; PMCID: PMC7878292. doi: 10.1016/j.ccell.2020.10.001.
28. Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019 Jan 1;30(1):44-56. PMID: 30395155; PMCID: PMC6336005. doi: 10.1093/annonc/mdy495.
29. Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell. 2018 May 14;33(5):843-852.e4. Epub 2018 Apr 12. PMID: 29657128; PMCID: PMC5953836. doi: 10.1016/j.ccell.2018.03.018.
30. Pender A, Titmuss E, Pleasance ED, Fan KY, Pearson H, Brown SD. Genome and Transcriptome Biomarkers of Response to Immune Checkpoint Inhibitors in Advanced Solid Tumors. Clin Cancer Res. 2021 Jan 1;27(1):202-212. Epub 2020 Oct 5. PMID: 33020056. doi: 10.1158/1078-0432.CCR-20-1163.
31. McGrail DJ, Pilié PG, Rashid NU, Voorwerk L, Slagter M, Kok M et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021 May;32(5):661-672 Epub 2021 Mar 15. PMID: 33736924; PMCID: PMC8053682. doi: 10.1016/j.annonc.2021.02.006.
32. Bhamidipati D, Subbiah V. Tumor-agnostic drug development in dMMR/MSI-H solid tumors. Trends Cancer. 2023 Oct;9(10):828-839. Epub 2023 Jul 28. PMID: 37517955. doi: 10.1016/j.trecan.2023.07.002.
33. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N Engl J Med. 2020 Dec 3;383(23):2207-2218. PMID: 33264544. doi: 10.1056/NEJMoa2017699.
34. San-Román-Gil M, Torres-Jiménez J, Pozas J, Esteban-Villarrubia J, Albarrán-Fernández V, Álvarez-Ballesteros P, Current Landscape and Potential Challenges of Immune Checkpoint Inhibitors in Microsatellite Stable Metastatic Colorectal Carcinoma. Cancers (Basel). 2023 Jan 30;15(3):863. PMID: 36765821; PMCID: PMC9913409. doi: 10.3390/cancers15030863.
35. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017 Jul 28;357(6349):409-413. Epub 2017 Jun 8. PMID: 28596308; PMCID: PMC5576142. doi: 10.1126/science.aan6733.
36. Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018 Sep;189:45-62.. Epub 2018 Apr 15. PMID: 29669262. doi: 10.1016/j.pharmthera.2018.04.004
37. Sukumar J, Gast K, Quiroga D, Lustberg M, Williams N. Triple-negative breast cancer: promising prognostic biomarkers currently in development. Expert Rev Anticancer Ther. 2021 Feb;21(2):135-148. PMID: 33198517; PMCID: PMC8174647. doi: 10.1080/14737140.2021.1840984.
38. Aguiar PN Jr, De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017 May;9(6):499-506 PMID: 28472902. doi: 10.2217/imt-2016-0150.
39. Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015 Apr;14(4):847-56. Epub 2015 Feb 18. PMID: 25695955. doi: 10.1158/1535-7163.MCT-14-0983.
40. Li Y, Meng Y, Sun H, Ye L, Zeng F, Chen X. The Prognostic Significance of Baseline Neutrophil-to-Lymphocyte Ratio in Melanoma Patients Receiving Immunotherapy. J Immunother. 2022 Jan 1;45(1):43-50. PMID: 34510106; PMCID: PMC8654256. doi: 10.1097/CJI.0000000000000392.
41. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil-to-lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta-analysis. Onco Targets Ther. 2018 Feb 23;11:955-965. PMID: 29503570; PMCID: PMC5827677. doi: 10.2147/OTT.S153290.
42. Takenaka Y, Oya R, Takemoto N, Inohara H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck. 2022 May;44(5):1237-1245. Epub 2022 Feb 10. PMID: 35146824. doi: 10.1002/hed.26997
43. Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017 Sep;111:176-181. Epub 2017 Jul 24. PMID: 28838390. doi: 10.1016/j.lungcan.2017.07.024.
44. Booka E, Kikuchi H, Haneda R, Soneda W, Kawata S, Murakami T. Neutrophil-to-Lymphocyte Ratio to Predict the Efficacy of Immune Checkpoint Inhibitor in Upper Gastrointestinal Cancer. Anticancer Res. 2022 Jun;42(6):2977-2987. PMID: 35641297. doi: 10.21873/anticanres.15781.