DISCOVERIES REPORTS (ISSN 2393249X), 2022, volume 5
REVIEW ARTICLE
CITATION: Hange N, Somagutta MR, Sharma A, Agadi K, Ngaba NN, Paikkattil N, Pormento MKLL. Latent Tuberculosis: Challenges and Opportunities for Diagnosis and Treatment. Discoveries Reports 2022; 5(1): e27. DOI: 10.15190/drep.2022.1
Latent Tuberculosis: Challenges and Opportunities for Diagnosis and Treatment
Namrata Hange1,*, Manoj Reddy Somagutta2, Amrit Sharma3, Kuchalambal Agadi4, Neguemadji Ngardig Ngaba5, Nidhin Paikkattil6, Maria Kezia Lourdes Ligsay Pormento7
(1) Eurasian Cancer Research Council, Mumbai, India
(2) Department of Medicine, Avalon University School of Medicine, Willemstad, Curaçao
(3) Department of Community Health, Christian Medical College, Vellore, Tamil Nadu, India
(4) Seema Dental College and Hospital, Rishikesh, Uttarakhand, India
(5) Faculté de Médecine Bon Samaritain of Walia, N'djamena, Chad
(6) Indian institute of Technology, Kharagpur, India
(7) Ateneo School of Medicine and Public Health, Quezon, Philippines
* Corresponding author: Dr. Namrata Hange, Eurasian Cancer Research Council, B - 1210, Golf Scappe, Diamond Garden, Basant Garden, Chembur, Mumbai, 400071, India; Phone: +917 709052487; drnamratah3@yahoo.co.in
Abstract
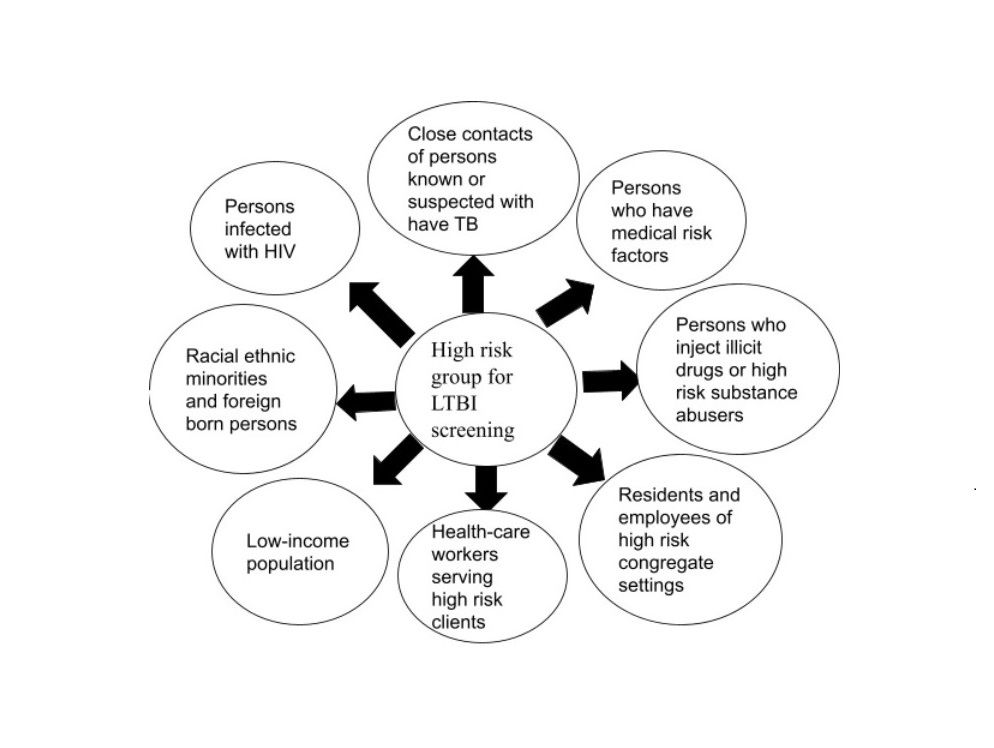
It is imperative to contain this silent epidemic of tuberculosis (TB), considering the monstrous burden of TB incident cases and deaths. As part of the WHO initiative of the end tuberculosis strategy, TB-preventive treatment (TPT) initiation to reduce TB incidence has been considered the ultimate preventive resort. This article addresses the challenges and strategies for latent TB management. Targeted LTBI Screening in the population with suspicion of TB infection with an effective contact screening strategy is an ideal preliminary step. Along with precise, short regimen treatment initiation for LTBI, the health care system ought to ensure the entire course of therapy. Effective utilization of existing infrastructure and resources for TB elimination is ideal for handling contact screening and LTBI management. Inconsistent guidelines about TB contact screening and cognizance of the population regarding attending an LTBI screening clinic are the first-line barriers hindering screening rates. Poverty, malnutrition, TB-HIV co-infection, long-term treatment, adverse effects, and associated out-of-pocket expenditure pose significant concerns for LTBI treatment. A cost-effective treatment regimen with a shorter duration and fewer adverse effects, population-specific treatment strategies with social intervention-teaching, and dedicated health care staff are crucial to ensuring the initiation and completion of LTBI treatment. Embracing LTBI strategy with active TB patient community engagement and education is essential for TB eradication across the globe. However, the most effective resort for TB elimination requires a specific LTBI management program. Until then, optimizing our current tools and strategies is essential for progress towards the TB elimination.
References
1. World Health Organization. Global tuberculosis report 2019. Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
2. World Health Organization: The End TB Strategy.2015; (accessed 10th June 2021). WHO, Geneva, Switzerland.
3. Uplekar M, Weil D, Lonnroth K, Jaramillo E, Lienhardt C, Dias HM, Falzon D, Floyd K, Gargioni G, Getahun H, Gilpin C, Glaziou P, Grzemska M, Mirzayev F, Nakatani H, Raviglione M; for WHO's Global TB Programme. WHO's new end TB strategy. Lancet. 2015 May 2;385(9979):1799-1801.
4. Tuberculosis: The Difference Between Latent TB Infection and TB Disease. Centers for Disease Control and Prevention. November 21, 2014.
5. Centers for Disease Control and Prevention.. Latent tuberculosis infection: a guide for primary health care providers. Atlanta, GA: CDC.Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. April 5, 2016.
6. TB Elimination The Difference Between Latent TB Infection and TB Disease, Division of Tuberculosis Elimination, National Center for HIV, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention, available on -https://www.cdc.gov/tb/publications/factsheets/general/LTBIandActiveTB.pdf, accessed on 11 Nov 2021.
7. Global tuberculosis report 2020. Geneva: World Health Organization; 2020. Licence: CC BY-NC-SA 3.0 IGO.
8. Cohen A, Mathiasen VD, Schön T, Wejse C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019 Sep 12;54(3):1900655.
9. Houben RM, Dodd PJ. The Global Burden of Latent Tuberculosis Infection: A Re-estimation Using Mathematical Modelling. PLoS Med. 2016 Oct 25;13(10):e1002152.
10. Knight GM, McQuaid CF, Dodd PJ, Houben RMGJ. Global burden of latent multidrug-resistant tuberculosis: trends and estimates based on mathematical modelling. Lancet Infect Dis. 2019 Aug;19(8):903-912.
11. WHO releases new global lists of high-burden countries for TB, HIV-associated TB and drug-resistant TB available at https://www.who.int/news/item/17-06-2021-who-releases-new-global-lists-of-high-burden-countries-for-tb-hiv-associated-tb-and-drug-resistant-tb, accessed at 1Oct 2021
12. Kiazyk S, Ball TB. Latent tuberculosis infection: An overview. Can Commun Dis Rep. 2017 Mar 2;43(3-4):62-66.
13. Colangeli R, Gupta A, Vinhas SA, Chippada Venkata UD, Kim S, Grady C et al. Mycobacterium tuberculosis progresses through two phases of latent infection in humans. Nat Commun. 2020 Sep 25;11(1):4870.
14. Sloot R, Schim van der Loeff MF, Kouw PM, Borgdorff MW. Risk of tuberculosis after recent exposure. A 10-year follow-up study of contacts in Amsterdam. Am J Respir Crit Care Med. 2014 Nov 1;190(9):1044-52.
15. TB Elimination Interferon-Gamma Release Assays (IGRAs) – Blood Tests for TB Infection, https://www.cdc.gov/tb/publications/factsheets/testing/igra.htm acessed on 1st Oct 2021
16. Centers for Disease Control. Screening for tuberculosis and tuberculosis infection in high-risk populations and the use of preventive therapy for tuberculous infection in the United States: recom mendations of the Advisory Committee for the Elimination of Tuberculosis. MMWR. 1990;39(8):1-2. https://www.cdc.gov/mmwr/preview/mmwrhtml/00038873.htm
17. Huaman MA, Sterling TR. Treatment of Latent Tuberculosis Infection-An Update. Clin Chest Med. 2019 Dec;40(4):839-848.
18. Redelman-Sidi G, Sepkowitz KA. IFN-γ release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med. 2013 Aug 15;188(4):422-31.
19. Swindells S, Ramchandani R, Gupta A, Benson CA, Leon-Cruz J, Mwelase N et al. One Month of Rifapentine plus Isoniazid to Prevent HIV-Related Tuberculosis. N Engl J Med. 2019 Mar 14;380(11):1001-1011.
20. Fox GJ, Dobler CC, Marais BJ, Denholm JT. Preventive therapy for latent tuberculosis infection-the promise and the challenges. Int J Infect Dis. 2017 Mar;56:68-7621.
21. Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of Latent Tuberculosis Infection: An Updated Network Meta-analysis. Ann Intern Med. 2017 Aug 15;167(4):248-255.
22. Comstock GW. How much isoniazid is needed for prevention of tuberculosis among immunocompetent adults? Int J Tuberc Lung Dis. 1999 Oct;3(10):847-50. PMID: 10524579.
23. Diallo T, Adjobimey M, Ruslami R, Trajman A, Sow O, Obeng Baah J et al. Safety and Side Effects of Rifampin versus Isoniazid in Children. N Engl J Med. 2018 Aug 2;379(5):454-463.
24. Cataño JC, Morales M. Follow-up results of isoniazid chemoprophylaxis during biological therapy in Colombia. Rheumatol Int. 2015 Sep;35(9):1549-5325.
25. Smith BM, Schwartzman K, Bartlett G, Menzies D. Adverse events associated with treatment of latent tuberculosis in the general population. CMAJ. 2011 Feb 22;183(3):E173-9.
26. Tang P, Johnston J. Treatment of Latent Tuberculosis Infection. Curr Treat Options Infect Dis. 2017;9(4):371-379.
27. World Health Organization Latent tuberculosis infection: updated and consolidated guidelines for programmatic management [Internet]. Geneva: World Health Organization; 2018. PMID: 30277688. Available from: http://www.who.int/tb/publications/latent-tuberculosis-infection/en/.Cited on October 02, 2021.
28. Kim HW, Kim JS. Treatment of Latent Tuberculosis Infection and Its Clinical Efficacy. Tuberc Respir Dis (Seoul). 2018 Jan;81(1):6-12.
29. Pineda NI, Pereira SM, Matos ED, Barreto ML. Chemoprophylaxis in the prevention of tberculosis. Jornal Brasileiro de Pneumologia. 2004;30:395-405.
30. Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F et al. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: results of an 11-year randomized study. Clin Infect Dis. 2007 Sep 15;45(6):715-22.
31. Njie GJ, Morris SB, Woodruff RY, Moro RN, Vernon AA, Borisov AS. Isoniazid-Rifapentine for Latent Tuberculosis Infection: A Systematic Review and Meta-analysis. Am J Prev Med. 2018 Aug;55(2):244-252.
32. Villarino ME, Scott NA, Weis SE, Weiner M, Conde MB, Jones B, et al. International Maternal Pediatric and Adolescents AIDS Clinical Trials Group; Tuberculosis Trials Consortium. Treatment for preventing tuberculosis in children and adolescents: a randomized clinical trial of a 3-month, 12-dose regimen of a combination of rifapentine and isoniazid. JAMA Pediatr. 2015 Mar;169(3):247-55.
33. Panayiotis D. Ziakas, Eleftherios Mylonakis. 4 Months of Rifampin Compared with 9 Months of Isoniazid for the Management of Latent Tuberculosis Infection: A Meta-analysis and Cost-Effectiveness Study That Focuses on Compliance and Liver Toxicity, Clinical Infectious Diseases, Volume 49, Issue 12, 15 December 2009, Pages 1883–1889.
34. Menzies D, Adjobimey M, Ruslami R, Trajman A, Sow O, Kim H et al. Four Months of Rifampin or Nine Months of Isoniazid for Latent Tuberculosis in Adults. N Engl J Med. 2018 Aug 2;379(5):440-453.
35. Nelson K. Tuberculin testing to detect latent tuberculosis in developing countries. Epidemiology. 2007 May;18(3):348-9.
36. Chee CBE, Reves R, Zhang Y, Belknap R. Latent tuberculosis infection: Opportunities and challenges. Respirology. 2018 Oct;23(10):893-900
37. Matteelli A, Sulis G, Capone S, D'Ambrosio L, Migliori GB, Getahun H. Tuberculosis elimination and the challenge of latent tuberculosis. Presse Med. 2017 Mar;46(2 Pt 2):e13-e21..
38. Jeon D. Latent tuberculosis infection: recent progress and challenges in South Korea. Korean J Intern Med. 2020 Mar;35(2):269-275.
39. Paton NI, Borand L, Benedicto J, Kyi MM, Mahmud AM, Norazmi MN et al. Diagnosis and management of latent tuberculosis infection in Asia: Review of current status and challenges. Int J Infect Dis. 2019 Oct;87:21-29.
40. Getahun H, Matteelli A, Abubakar I, Aziz MA, Baddeley A, Barreira D et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015 Dec;46(6):1563-76.
41. Pradipta IS, Houtsma D, van Boven JFM, Alffenaar JC, Hak E. Interventions to improve medication adherence in tuberculosis patients: a systematic review of randomized controlled studies. NPJ Prim Care Respir Med. 2020 May 11;30(1):21.
42. Menzies D, Alvarez G, Khan K. Treatment of latent tuberculosis infection. In: Canadian tuberculosis standards. 7th ed. Ottawa: Public Health Agency of Canada; 2014 https://www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition/edition-18.html
43. Gengiah TN, Gray AL, Naidoo K, Karim QA. Initiating antiretrovirals during tuberculosis treatment: a drug safety review. Expert Opin Drug Saf. 2011 Jul;10(4):559-74.
44. Dheda K, Shean K, Zumla A, Badri M, Streicher EM, Page-Shipp L et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010 May 22;375(9728):1798-807.
45. Seung KJ, Keshavjee S, Rich ML. Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Cold Spring Harb Perspect Med. 2015 Apr 27;5(9):a017863.
46. Chakaya J, Khan M, Ntoumi F, Aklillu E, Fatima R, Mwaba P et al. Global Tuberculosis Report 2020 - Reflections on the Global TB burden, treatment and prevention efforts. Int J Infect Dis. 2021 Mar 11:S1201-9712(21)00193-4.
47. Understanding Poverty, World bank, Available at: https://www.worldbank.org/en/topic/poverty. Accessed on:8 Aug 2021.
48. Poverty & Equity Brief India South Asia October 2020, Poverty & Equity Brief India South Asia April 2021. Accessed on 8th Aug 2021. Available at:
https://databank.worldbank.org/data/download/poverty/987B9C90-CB9F-4D93-AE8C-750588BF00QA/SM2020/Global_POVEQ_IND.pdf .
49. Malnutrition, fact sheet, WHO accessed at https://www.who.int/news-room/fact-sheets/detail/malnutrition on 8th Aug 2021.
50. Webb P, Stordalen GA, Singh S, Wijesinha-Bettoni R, Shetty P, Lartey A. Hunger and malnutrition in the 21st century. BMJ. 2018 Jun 13;361:k2238.
51. Semba RD, Darnton-Hill I, De Pee S. Addressing tuberculosis in the context of malnutrition and HIV coinfection. Food and Nutrition Bulletin. 2010 Dec;31(4_suppl4):S345-64.
52. Knut Lönnroth, Brian G Williams, Peter Cegielski, Christopher Dye, A consistent log-linear relationship between tuberculosis incidence and body mass index, International Journal of Epidemiology, Volume 39, Issue 1, February 2010;149–155.
53. Daniel J Carter, Philippe Glaziou, Knut Lönnroth, Andrew Siroka, Katherine Floyd, Diana Weil, Mario Raviglione, Rein M G J Houben, Delia Boccia, The impact of social protection and poverty elimination on global tuberculosis incidence: a statistical modelling analysis of Sustainable Development Goal 1, The Lancet Global Health, Volume 6, Issue 5, 2018; e514-e522, ISSN 2214-109X
54. Loureiro RB, Maciel ELN, Caetano R, Peres RL, Fregona G, Golub JE, et al. Cost-effectiveness of QuantiFERON-TB Gold In-Tube versus tuberculin skin test for diagnosis and treatment of Latent Tuberculosis Infection in primary health care workers in Brazil. PLoS ONE ,2019, 14(11): e0225197
55. Paton NI, Borand L, Benedicto J, Kyi MM, Mahmud AM, Norazmi MN et al. Diagnosis and management of latent tuberculosis infection in Asia: review of current status and challenges. International Journal of Infectious Diseases. 2019 Oct 1;87:21-9.
56. Loureiro RB, Maciel ELN, Caetano R, Peres RL, Fregona G, Golub JE, Braga JU. Cost-effectiveness of QuantiFERON-TB Gold In-Tube versus tuberculin skin test for diagnosis and treatment of Latent Tuberculosis Infection in primary health care workers in Brazil. PloS one, 2019;14(11), e0225197.
57. Global Drug Facility.July 2020 medicines catalog. Geneva, Switzerland Stop TB Partnership 2020 Available at: http://www.stoptb.org/assets/documents/gdf/drugsupply/GDFMedicinesCatalog.pdf. Accessed 20 July 2020.
58. Meeting of the Implementation Core Group of WHO Global Task Force on Latent TB Infection and country stakeholders on implementation tools and joint TB and HIV programming to scale up TB preventive treatment Nov 2018 https://www.who.int/tb/publications/Meeting_Implementation_Core_Group_WHO_Global_Task_Force_Latent_TB_Infection.pdf
59. Liu Y, Birch S, Newbold KB, Essue BM. Barriers to treatment adherence for individuals with latent tuberculosis infection: a systematic search and narrative synthesis of the literature. The International journal of health planning and management. 2018 Apr;33(2):e416-33.